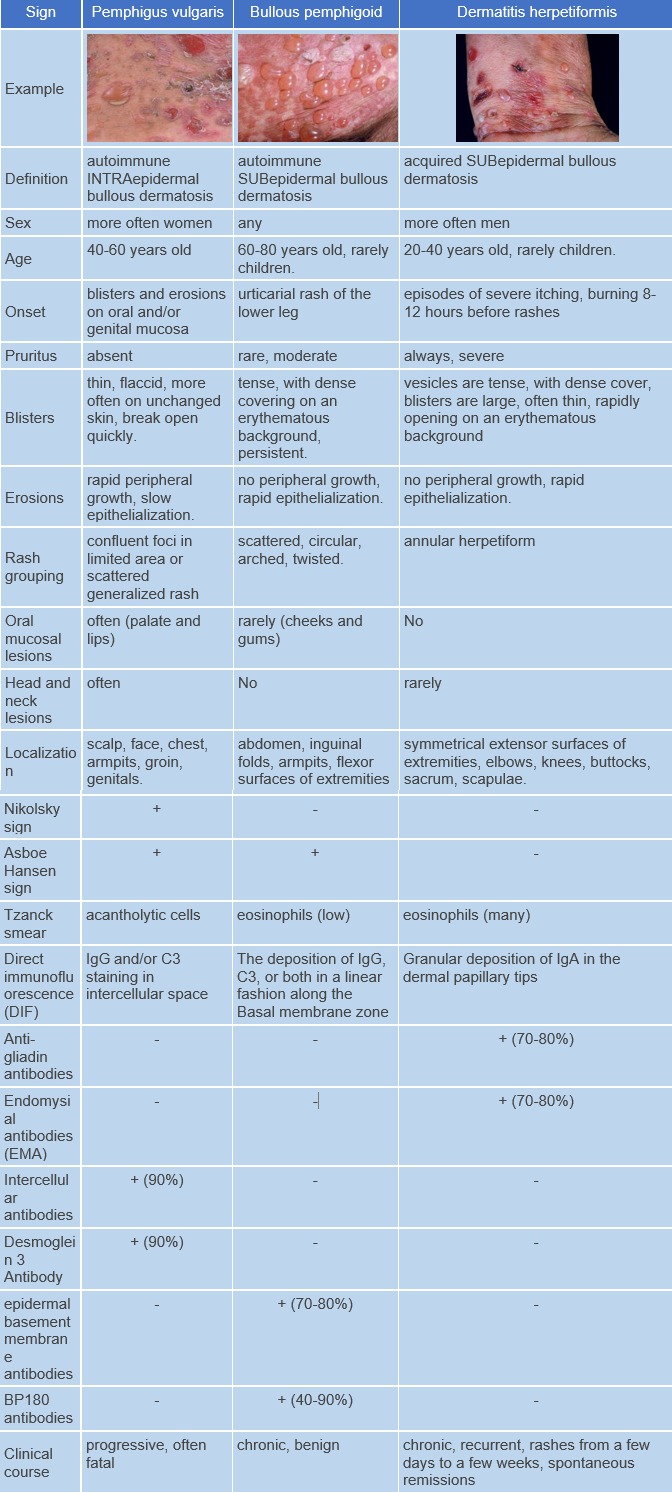
Bullous pemphigoid is an autoimmune skin disease characterized by the production of autoantibodies against components of hemidesmosomes (BP180 and BP230 antigens) and the formation of subepidermal blisters. ICD-10 Code: L12.0
In most cases, the development of bullous pemphigoid is not associated with a specific triggering factor. However, in some patients, the appearance of lesions is associated with the use of certain medications, exposure to physical factors, or viral infections.
Medications that have been associated with the development of bullous pemphigoid include penicillamine, penicillins, cephalosporins, captopril and other angiotensin-converting enzyme inhibitors, furosemide, aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), and nifedipine. Cases of bullous pemphigoid have also been reported following influenza vaccination and tetanus toxoid vaccination. The development of bullous pemphigoid has been described following exposure to physical factors such as ultraviolet radiation, radiation therapy, thermal and electrical burns, and surgical procedures. Viral infections (such as hepatitis B and C viruses, cytomegalovirus, Epstein-Barr virus) have also been implicated in the development of bullous pemphigoid.
The development of bullous pemphigoid is triggered by the production of IgG autoantibodies against BP180 (type XVII collagen) and BP230 proteins, which are structural components of the basement membrane of the skin.
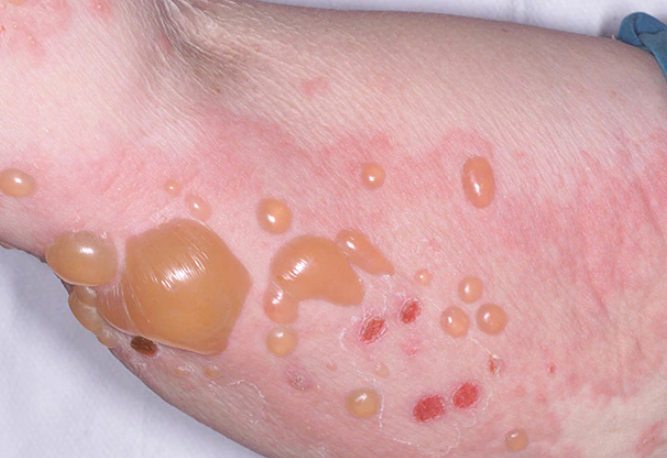
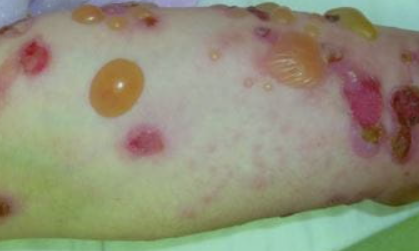
Skin involvement in bullous pemphigoid may be localized or generalized. Lesions are more commonly localized on the extremities, abdomen, groin-thigh folds, and inner surface of the thighs.
The skin lesions in bullous pemphigoid may be polymorphic. The disease typically begins with the appearance of erythematous, papular, and/or urticarial-like eruptions accompanied by pruritus. These eruptions may persist for several months before developing into blisters.
The blisters are tense, firm and round or oval in shape. They contain serous or serosanguinous fluid and may appear on an erythematous background or on uninvolved skin. Erosions may form at the site of the blisters and, in the absence of secondary infection, they epithelialize rapidly and do not tend to spread peripherally. The Nikolsky sign is negative. Mucous membranes are involved in 10-25% of cases. The disease is characterized by a chronic recurrent course.
The severity of bullous pemphigoid is determined by the number of blistering lesions. It is considered severe when more than 10 blisters appear daily for three consecutive days and mild when 10 or fewer blisters appear daily.
The goal of treatment for bullous pemphigoid is to achieve remission. The following factors should be considered when prescribing and administering therapy to patients with bullous pemphigoid:
- Potential patient comorbidities (such as diabetes mellitus, hypertension, ischemic heart disease, neurologic disorders)
- Adverse effects associated with systemic and topical therapies
During treatment with systemic glucocorticosteroids, blood pressure should be monitored to assess cardiovascular health and blood glucose levels should be controlled.
During cytostatic therapy, hemoglobin, leukocyte, and platelet counts, liver and kidney function parameters, and urinalysis should be monitored. When systemic glucocorticosteroids and immunosuppressants are administered, signs of infectious diseases and complications should also be promptly identified.
Treatment Regimens:
For mild bullous pemphigoid:
Clobetasol propionate 0.05% applied topically once daily to the affected areas. After 15 days of clinical improvement (no new eruptions, itching stops, and erosions begin to epithelialize), gradually reduce the amount of topical glucocorticosteroid applied.
If there is no clinical response to topical glucocorticosteroid therapy within 1-3 weeks:
Oral prednisone at a dose of 0.5 mg per kg of body weight per day. Once clinical improvement is achieved, gradually reduce the dose of prednisolone to 0.1 mg per kg of body weight per day. The duration of therapy is 4-12 months.
For severe bullous pemphigoid:
Clobetasol propionate 0.05% applied topically once daily to the affected areas. After 15 days of clinical improvement (no new eruptions, itching stops, and erosions begin to epithelialize), gradually reduce the amount of topical glucocorticosteroid applied. In addition, administer oral prednisolone in a dose of 0.5-0.75 mg per kg of body weight, depending on the severity of the condition. Effectiveness of prednisolone is insufficient if prescribed in a dose less than 0.5 mg per kg of body weight. Increasing the dose of prednisolone above 0.75 mg per kg of body weight does not increase the effectiveness of therapy. Gradual tapering of the systemic corticosteroid dose should begin after clinical improvement is achieved and should be continued for 4-6 months until a maintenance dose of 0.1 mg per kg of body weight per day is reached. If the patient remains in clinical remission for 3-6 months, treatment can be discontinued. In case of relapse, the corticosteroid dose should be increased to the initial level.
If it becomes necessary to reduce the dose of systemic corticosteroids, the following options may be prescribed:
- Azathioprine: 2 mg per kg of body weight per day for 3-4 weeks in combination with prednisolone at a dose of 0.5 mg per kg of body weight per day. Prescribing azathioprine at a dose of 100-150 mg per day in combination with prednisone at a dose of 1 mg per kg of body weight per day does not increase the efficacy of bullous pemphigoid therapy compared to monotherapy with prednisone at a dose of 1 mg per kg of body weight per day, but increases the number of treatment-related adverse effects.
- Mycophenolate mofetil: 1000 mg twice a day (2000 mg per day) orally for 6 weeks in combination with prednisone at a dose of 0.5 mg per kg of body weight per day.
- Methotrexate: 5-15 mg per week orally or intramuscularly, dose to be increased or decreased based on efficacy and tolerability, in combination with clobetasol propionate applied topically twice daily to the entire body surface except the face for 3 weeks, followed by a gradual reduction in the daily dose of clobetasol propionate over 12 weeks, and then maintenance therapy with methotrexate at a dose of 10 mg per week as monotherapy for 4-12 months.
- Cyclophosphamide: 50 mg orally per day, increasing to 100 mg per day if inadequate response.
Treatment outcomes should include:
- Stop the progression of the disease.
- Reduce itching.
- Healing of erosions.
There are no specific preventive methods for bullous pemphigoid.