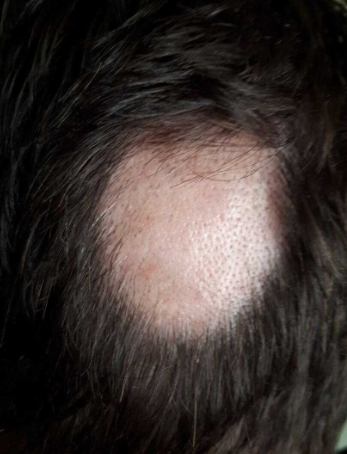
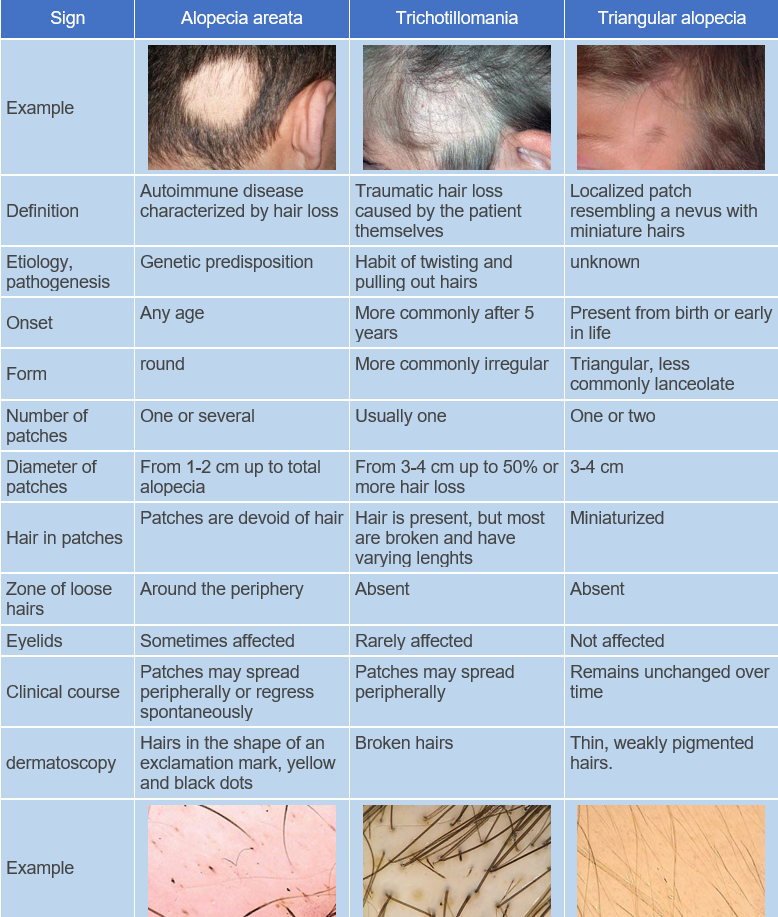
Alopecia areata is a chronic organ-specific autoimmune inflammatory disease with a genetic predisposition, characterized by the involvement of hair follicles and, in some cases (in 7-66% of patients), the nails, resulting in persistent or temporary non-scarring hair loss. ICD-10 Code: L63.
The development of the disease is thought to involve a local autoimmune mechanism that damages the hair follicle, leading to a disruption of immune tolerance to the follicular cells. The incidence and prevalence of alopecia areata varies according to geographical and ethnic differences, as well as the immunogenetic background of the patients. Both males and females are susceptible to the disease. Predisposition to the disease is genetic, with 10-20% of patients having a family history of the condition, and the true frequency of the disease is likely higher, as mild cases may go unnoticed. The genetic predisposition is polygenic in nature and is associated with specific HLA class II alleles, in particular dQB103 and dRB11104. The HLA alleles dQB10301 (HLA-dQ7) and dRB11104 (HLA-dR11) may be associated with total and universal alopecia. Triggers of the disease may include stress, vaccinations, viral and infectious diseases, use of antibacterial agents, anesthesia, and others.
Diseases Associated with Alopecia Areata:
Autoimmune thyroid disease is seen in 8-28% of patients, although the presence of thyroid antibodies in the blood does not correlate clinically with severity. Vitiligo is seen in 3-8% of patients. Atopy is twice as common in patients as in the general population.
Relatives of patients have an increased risk of developing type 1 diabetes, although the incidence in patients themselves may be lower than in the general population. Patients often experience high levels of mental disorders, particularly anxiety and depressive disorders.
The incidence is estimated at 0.7-3.8% of dermatology patients seeking medical care. The lifetime risk of developing the disease is 1.7%. The condition affects men and women equally. The first patch of hair loss appears in childhood in 20% of patients, in persons under 20 years of age in 60% of patients, and in persons over 40 years of age in 20% of patients.
Classification:
- L63.0 Alopecia totalis
- L63.1 Alopecia universalis
- L63.2 Alopecia areata, reticular form
- L63.8 Other forms of alopecia areata
Depending on the extent and type of hair loss, the following clinical forms are distinguished:
- Localized (limited) alopecia.
- Subtotal alopecia.
- Total alopecia.
- Universal alopecia.
Other forms include:
- Multifocal (reticulate) alopecia.
- Ophiasis.
- Inverse ophiasis (sisapho).
- Diffuse form.
In localized (limited) form, one or several well-defined round patches of alopecia are present on the hairy part of the scalp.
In subtotal form, more than 40% of the hair is missing from the hairy part of the scalp. In ophiasis, the patches of alopecia have a band-like shape and affect the entire border zone of hair growth in the occipital and temporal areas.
In inverse ophiasis (sisapho), the band-like patches of alopecia extend to the frontal-temporal and occipital areas.
Diffuse form is characterized by partial or complete diffuse thinning of the hair on the hairy part of the scalp.
In total form, there is complete loss of terminal hair on the hairy part of the scalp.
In universal form, hair is absent not only from the hairy part of the scalp, but also from the eyebrow and eyelash areas and from the skin of the trunk.
Stages of the pathological process:
Active (progressive) stage: Subjective symptoms are usually absent, although some patients may complain of itching, burning, or pain in the affected areas. Typical patches of non-scarring alopecia are round or oval with no change in skin color. Less commonly, moderately red or peach-colored patches may be observed. Proximally narrowed and distally widened hairs in the shape of exclamation points are a characteristic feature often seen on or around the affected area. In the active phase of the disease, a "pull test" on the hairs at the margins of the lesions, known as the "hair pull test zone," may be positive. The border of the zone does not exceed 0.5-1 cm. Alopecia can spread to almost any area of the hair-bearing skin, but about 90% of patients have scalp involvement. In the initial stage, gray hair is not affected.
Stationary stage: Around the alopecia patch, the "hair pulling test" zone is not observed and the skin within the patch remains unchanged.
Regressive stage: Vellus hairs - small, fine, depigmented hairs - and partially regrowing pigmented terminal hairs may be seen in the alopecia patch. When hair regrowth resumes, the initial hairs are usually hypopigmented, but their color typically returns over time.
Patients may also have specific dystrophic changes in the nails: punctate nail pits, trachyonychia, Beau's lines, onychorrhexis, nail thinning or thickening, onychomadesis, koilonychia, punctate or transverse leukonychia, and red lunulae.
Up to 50% of patients may recover within a year without treatment (spontaneous remission). Of those affected, 85% may experience more than one episode of the disease. If the disease occurs before puberty, there is a 50% chance of developing total alopecia. The probability of complete recovery in cases of total/universal alopecia is less than 10%.The diagnosis is based on the clinical presentation of the disease, which includes the following characteristics:
- Presence of well-defined patches of alopecia on the skin.
- Presence of exclamation mark hairs and a "hair pull test zone" at the border of the alopecia patch (active stage).
- Microscopic examination of epilated hairs from the border zone of the patch reveals dystrophic hair ends resembling "beaded hair."
- Presence of light-colored vellus hairs in the alopecia patch (regressive stage), sometimes with exclamation mark hairs on one edge of the patch and vellus hair growth on the opposite edge.
- Specific dystrophic nail changes observed during nail examination.
- Trichoscopy (dermoscopy of the scalp) may reveal "yellow dots," cadaverized hairs, and hairs in the shape of exclamation marks.
In cases of uncertain diagnosis or before initiating treatment, the following laboratory investigations are recommended:
- Microscopic examination of skin and hair for the presence of pathogenic fungi.
- Microscopic examination of epilated hairs from the edge of the alopecia patch to identify dystrophic hair ends, a pathognomonic sign of alopecia.
- Histological examination of a skin fragment from the scalp
- Complete blood count.
- Serological tests to rule out conditions like systemic lupus erythematosus (SLE) and syphilis.
- Cortisol level in the blood (before and 4 weeks after planning treatment with systemic glucocorticoids).
- Blood biochemistry, including ALT, AST, total protein, bilirubin, cholesterol, blood sugar, and alkaline phosphatase (if toxic alopecia is suspected or before initiating phototherapy with internal photosensitizers).
- Skull X-ray to rule out space-occupying lesions in the area of the sella turcica.
- Blood hormone tests (free T3, free T4, TSH, anti-TPO antibodies, anti-TG antibodies) to rule out thyroid pathology, and prolactin level to rule out hyperprolactinemia.
- Rheoencephalography (REG) is recommended for children under 12 years of age with extensive forms to diagnose possible cerebral vascular circulation disorders.
- Consultations with other specialists such as a neurologist, endocrinologist, and psychotherapist may be recommended based on individual indications.
Systemic therapy for severe forms
Glucocorticosteroid drugs
- Prednisone 200 mg per week orally for 3 months
- Prednisone starting at 40 mg per day orally (with gradual dose reduction) for 6 weeks
- Prednisone 80-100 mg per day orally for 3 consecutive days every 3 months
- Methylprednisone 250 mg twice a day intravenously for 3 consecutive days every 3 months
Antimetabolites
- Methotrexate 15-30 mg once a week orally or subcutaneously for 9 months; if a positive effect is achieved, therapy can be extended to 18 months, if no positive effect, methotrexate should be discontinued
- Methotrexate 15-30 mg once a week orally or subcutaneously in combination with prednisolone 10-20 mg per day orally until hair regrowth resumes.
Immunodepressants
- Cyclosporine (C) 2.5-6 mg per kg of body weight per day orally for 2-12 months. When a positive clinical result is achieved, the dose is gradually reduced until complete withdrawal.
Systemic therapy for localized (limited) form
- Zinc sulfate 5 mg per kg of body weight, 3 times a day orally after meals for 3 months.
Topical therapy for severe forms
- Minoxidil solution 5%, applied externally twice a day under an occlusive dressing until hair regrowth resumes.
- Clobetasol propionate ointment 0.05%, applied externally twice a day under an occlusive dressing, with a duration of therapy up to 2 months.
Topical therapy for localized (limited) form
Intralesional injection of glucocorticosteroids:
- Triamcinolone acetonide, administered every 4-6 weeks in the form of multiple intracutaneous injections, 0.5-1 cm apart and 0.1 ml using a 30-gauge needle. The maximum dose of triamcinolone acetonide per session should not exceed 20 mg. To reduce pain during the injections, a local anesthetic is applied prior to the procedure. If no positive effect is observed within 6 months of starting treatment, intralesional injections should be discontinued. Side effects may include temporary atrophy and telangiectasias. To reduce the risk of adverse effects, the volume and number of injections to the affected area should be reduced and intraepidermal injections should be avoided.
- Betamethasone dipropionate (2 mg) + betamethasone sodium phosphate (5 mg), intracutaneous injection in the affected area at a rate of 0.2 ml/cm2. The injection is evenly distributed using a tuberculin syringe and a 25-gauge needle. The drug is injected every 4 weeks, and the total amount of drug injected in all areas should not exceed 2 ml in 2 weeks.
Minoxidil:
- Minoxidil solution 2%, applied twice a day under an occlusive dressing until hair regrowth resumes.
- Minoxidil solution 5%, applied twice a day under an occlusive dressing until hair regrowth resumes.
Topical glucocorticosteroids:
- Fluocinolone acetonide, cream 0.25%, applied twice a day with a duration of therapy up to 2 months.
- Betamethasone valerate, foam 0.1%, cream applied twice a day with a duration of therapy up to 2 months.
- Betamethasone dipropionate, lotion 0.05%, cream applied twice a day with a duration of therapy up to 2 months.
- Clobetasol propionate, cream 0.05%, applied twice a day under an occlusive dressing with a duration of therapy up to 2 months.
- Hydrocortisone butyrate, cream 0.1%, emulsion applied twice a day with a duration of therapy up to 2 months.
- Mometasone furoate, cream 0.1%, lotion applied twice a day with a duration of therapy up to 2 months.
Prostaglandin F2a analogs for eyelash growth in eyelash alopecia:
- Latanoprost, solution 0.03%, applied daily in the evening to the base of the upper eyelashes for at least 1 month until clinical improvement is achieved.
- Bimatoprost, solution 0.03%, applied daily in the evening to the base of the upper eyelashes for at least 1 month until clinical improvement is achieved.
Light therapy:
For localized form: narrowband phototherapy with an excimer laser at a wavelength of 308 nm. The initial dose of laser irradiation is 50 mJ/cm2 below the minimum erythema dose; subsequent irradiation increases by 50 mJ/cm2 every two sessions. The affected area is treated twice a week for a total of no more than 24 sessions per course. For severe forms: PUVA therapy with psoralen and its derivatives at a dose of 0.5 mg per kg of body weight 2 hours before the procedure. The irradiation dose is gradually increased from 1 J per 1 cm2 to 15 J per 1 cm2.
Treatment outcome requirements: Hair regrowth in alopecic patches.
Treatment failure: Patients with prolonged absence of eyebrows may be offered dermatography or medical tattooing. Hair prostheses, wigs and hairpieces are recommended for patients with alopecia during the treatment period or in case of treatment failure. There are no preventive methods.