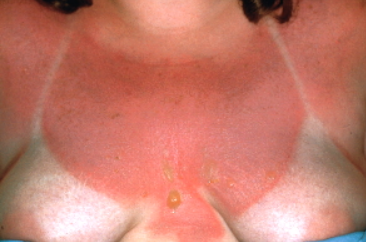
Drug-induced phototoxic reaction is an inflammatory response that occurs in the skin after exposure to sunlight or ultraviolet radiation in combination with a drug or chemical substance, resulting in photochemical damage to cellular structures. ICD-10 Code: L56.0
Phototoxic reactions occur in individuals of all races regardless of gender, and they are more common in adults than in children. Typically, they develop after exposure to long-wave ultraviolet radiation (UVA), less frequently to midwave ultraviolet radiation (UVB), or to visible light.
Phototoxic reactions can occur in anyone and are dose-dependent. When light photons interact with a drug or chemical substance in the skin, a photochemical reaction occurs, resulting in the formation of free radicals or reactive oxygen species that cause damage to cellular structures. The severity of a phototoxic reaction depends on the properties of the drug, such as absorption, metabolism, stability and solubility.
The following drugs and chemicals are the most common causes of phototoxic reactions:
- Systemic drugs: antibacterial agents (tetracyclines, quinolones), antiarrhythmic agents (amiodarone, quinidine), diuretics (furosemide, thiazides), alprazolam, antifungal agents (griseofulvin, itraconazole, voriconazole), furanocoumarin-containing medications, non-steroidal anti-inflammatory drugs (piroxicam, naproxen, ketoprofen), phenothiazines, sulfonylurea derivatives, isotretinoin, sulfonamide medications, calcium channel blockers, hypericin, photosensitizing agents used in photodynamic therapy (photofrin, foscan).
- Topical medications, chemical compounds, and plants causing phototoxic reactions: ketoprofen, dyes (methylene blue, eosin), tar and its components, furanocoumarin-containing medications, benzocaine, benzoyl peroxide, ingredients in sunscreens (benzophenones, derivatives of para-aminobenzoic acid), components of cosmetic products (aromatic substances, essential oils of bergamot, lime, sandalwood, lemon, and cedar), preservatives, giant hogweed, St. John's wort, parsley, celery, parsnip, lime, lemon, fig, some meadow herbs.
Clinical presentation of phototoxic reactions is typically similar to sunburn: affected areas of skin develop erythema, swelling, blisters, and less commonly vesicles or bullae, often accompanied by itching, burning, tingling, or tenderness. After resolution of inflammatory symptoms, peeling and/or persistent skin hyperpigmentation may occur. Phototoxic reactions occur within a few hours or days after a drug interacts with sunlight (ultraviolet radiation) and last for several days or weeks. There are three types of phototoxic reactions:
- Immediate response: Erythema and urticaria
- Delayed sunburn-like reaction, which develops after 16-24 hours or later (if the photosensitizing agent is a psoralen, after 48-72 hours).
- Delayed melanin hyperpigmentation (appears after 72-96 hours)
The use of medications such as amiodarone and tricyclic antidepressants may lead to the development of gray-blue skin pigmentation. In some cases, desquamation of the epidermis may result in skin dyschromia.
Phototoxic reactions caused by doxycycline, tetracycline, fluoroquinolones, quinine, furanocoumarins, and certain other drugs may manifest as nail plate opacities, subungual hyperkeratosis, and onychohysis (photoonycholysis). Less commonly, pseudoporphyria may occur with clinical features resembling late cutaneous porphyria. Lichenoid phototoxic reactions have been described as well as reactions leading to the formation of telangiectasias.The diagnosis is based on the patient's history (report of use of any medication or chemical substance followed by sun exposure or artificial ultraviolet light exposure, appearance of eruptions on exposed skin areas), clinical presentation, results of phototesting, and laboratory tests.
Laboratory Tests:
- Complete blood count, urinalysis and blood biochemistry;
- Blood level autoantibodies: antinuclear factor, antibodies to double-stranded (native) DNA, Sm, Ro/SS-A and La/SS-B antigens, etc. (performed to rule out systemic lupus erythematosus);
- Porphyrin levels in blood plasma, erythrocytes, and urine (performed to rule out porphyria).
- Sunburn
- Irritant contact dermatitis
- Allergic contact dermatitis
- Eczema
- Systemic lupus erythematosus
- Porphyria cutanea tarda
Treatment Objectives
- Resolution of skin eruptions;
- Alleviation of negative subjective sensations; prevention of new eruptions;
- Improvement in the quality of life of patients.
General treatment features:
- The primary focus of treatment is to eliminate exposure to the drug or chemical that caused the adverse effect and to limit exposure to sunlight (ultraviolet). If complete discontinuation of the medication is not possible, it is recommended that the medication be taken in the evening to reduce the concentration of the drug in the skin during daylight hours. Patients should avoid sun exposure, especially during the most active hours of sunlight. Regular use of sunscreen and wearing clothing and hats are recommended.
- It should be noted that in some cases increased photosensitivity may persist for several weeks or months after discontinuation of the photosensitizing medication.
- Symptomatic therapy is used to treat eruptions. If blisters are present, they are opened. Cold compresses or moist, drying dressings are applied to areas of erythema and skin swelling.
- Use of moisturizers and topical glucocorticosteroids is recommended, although evidence of their efficacy is lacking. For acute inflammatory manifestations, glucocorticosteroids are prescribed in form of emulsions, lotions, or creams.
- In some cases, antihistamines or analgesics may be recommended.
- For severe reactions, systemic glucocorticosteroids are used.
Systemic Therapy
Antihistamines
- Loratadine 5-10 mg orally once daily for 7-10 days or
- Cetirizine 5-10 mg orally once daily for 7-10 days or
- Ebastine 5-20 mg orally once daily for 7-10 days or
- Levocetirizine 5 mg orally once daily for 7-10 days.
- Prednisone 60-80 mg per day orally for several days, followed by gradual tapering of the dose until complete discontinuation.
Topical Therapy
Topical Glucocorticosteroids
- Mometasone furoate topically 1-2 times a day or
- Methylprednisolone aceponate topically 1-2 times a day or
- Alclometasone dipropionate topically 1-2 times a day or
- Betamethasone valerate topically 1-2 times a day or
- Betamethasone dipropionate topically 1-2 times a day or
- Fluticasone propionate topically 1-2 times a day or
- Hydrocortisone butyrate topically 1-2 times a day or
- Clobetasol propionate topically 1-2 times a day.
Course: 2-4 weeks.
If topical therapy does not have the desired effect, systemic glucocorticosteroids are prescribed.
Prevention
- Patients should avoid the use of medications and chemicals that induce photoallergic reactions.
- If discontinuation of medication is not possible, patients should minimize their exposure to the sun, protect their skin from sunlight with clothing and SPF.
- Patients should be informed about the ability of UVA ultraviolet radiation to penetrate window glass.