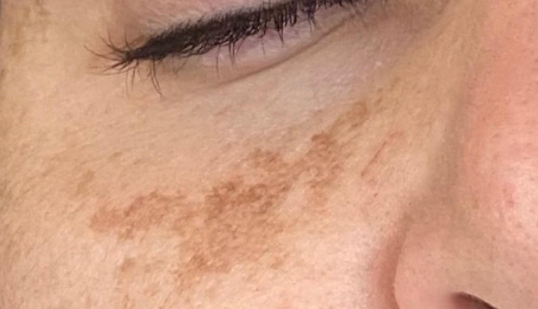
Melasma is an acquired facial hypermelanosis, appearing as irregularly shaped brown or gray-brown spots. ICD-10 code: L81.1.
Epidemiologic studies on the prevalence of melasma have not been published. Limited information is also due to the fact that many patients prefer to use over-the-counter whitening products rather than see a dermatologist. Although melasma predominantly affects women, men are not exempt, accounting for approximately 10% of melasma patients. People of all ethnic backgrounds are affected, although melasma is more common in people with darker skin tones of Spanish, East Asian and Indo-Chinese origin who live in areas with intense sunlight. In these cases, especially in women, melasma appears at a relatively early age.
The exact cause of melasma is unknown. The onset of the condition has been linked to factors such as sun exposure and genetic predisposition. The etiology and pathogenesis of this condition are believed to involve multiple factors. Natural and synthetic hormones such as estrogen and progesterone are thought to play a role in the pathogenesis, as evidenced by the association of melasma with pregnancy, oral contraceptive use, and ovarian tumors. Extensive studies of endocrinological parameters in melasma patients have shown elevated levels of luteinizing hormone (LH) and decreased levels of estradiol in serum. These abnormalities are suggestive of mild subclinical ovarian dysfunction. Similarly, male melasma patients show deviations from normal hormonal profiles, with elevated circulating LH levels and decreased serum testosterone levels. While the mechanism by which estrogen influences melasma exacerbation is unknown, it has been reported that melanocytes contain estrogen receptors that stimulate hyperactivity of these cells.
The use of cosmetic products containing certain components (oxidized linoleic acid, salicylates, preservatives, etc.), certain medications (anticonvulsants, etc.), as well as photosensitizing agents are often considered as etiological factors.
As mentioned above, the two most important causative factors of the disease are sun exposure and genetic predisposition. Sun exposure plays a crucial role. Melasma exacerbations are almost inevitable after uncontrolled sun exposure, and conversely, melasma resolves when sun exposure is avoided. Genetic and racial factors also dominate, as evidenced by familial cases of the disease and its greater prevalence in individuals of Spanish, East Asian and Chinese descent.- The epidermal type is characterized by the intensity of color contrast between melasma and normal skin.
- In the dermal type, the pigmentation of the epidermis does not intensify under Wood's lamp, and the contrast between the affected areas and unaffected skin becomes less obvious.
- In the mixed type, some areas in the same patient become more prominent under the Wood's lamp, while others become less prominent.
- Post-inflammatory hyperpigmentation: Localization of eruptions, history of inflammation.
- Pigmented cosmetic contact dermatitis: Reddish-brown pigmentation, reticular pattern
- Hyperthyroidism: Thyroid function tests.
- HIV infection: Serology.
- Phenytoin use: Medical history.
Melasma is a cosmetic problem that sometimes causes significant emotional distress. Currently, there is no universally effective treatment for melasma. Most existing methods can temporarily lighten melasma, but the condition typically recurs. However, there are several therapeutic modalities that may offer significant benefits. Before attempting to treat melasma, it's important to consider factors related to the abnormal pigmentation.
The goals of melasma therapy should be: a) to slow melanocyte proliferation, b) to slow melanosome formation, and c) to increase melanosome clearance.
General therapeutic recommendations:
Sun exposure should be avoided. Broad-spectrum sunscreens should be used as melanocytes in melasma are easily stimulated not only by ultraviolet B (UVB) but also by UVA and visible light. Sunscreen should be applied daily during treatment and indefinitely after discontinuation during the sunny months of the year. Tanning is absolutely contraindicated as a few minutes of sun exposure can destroy the results of months of treatment.
Women with melasma who are pregnant should avoid sun exposure during this time and use broad-spectrum sunscreen daily. Patients are advised to be patient as melasma may fade or resolve spontaneously within a few months after pregnancy.
Patients using oral contraceptives should discontinue use.
Hydroquinone (HQ)
Among the many substances used at various times to treat melasma, HQ is the most effective. The depigmenting action of HQ is based on the following properties.
HQ slows down melanin biosynthesis by inhibiting the conversion of tyrosine to melanin. HQ inhibits the formation of melanosomes or enhances their degradation, or both. HQ inhibits DNA and RNA synthesis in melanocytes. The efficacy of HQ is directly related to the concentration of the formulation, the composition of the vehicle and the chemical stability of the final product. Concentrations of HQ range from 2% (OTC product) to 10%, with the latter concentration being prescribed ex tempore in refractory cases. Such ex tempore HQ preparations often show an effect in patients who did not respond to lower concentrations. With controlled use and monitoring, the side effects of these preparations are minimal. The most appropriate vehicle for the drug formulation is either an ethanol-propylene glycol solution (equal parts propylene glycol and absolute ethanol), a hydrophilic ointment, or a gel containing 10% alpha-hydroxy acids (AHAs). The chemical stability of the HQ formulation is critical. HQ is susceptible to oxidation and loses efficacy. Therefore, antioxidants such as 0.1% sodium bisulfite and 0.1% ascorbic acid are required to maintain formulation stability.
Considering the desired HQ concentration, vehicle and chemical stability, the following formulation can be prescribed:
- HQ-10%;
- In ethanol and propylene glycol 1:1 (or based on a cream or gel with 10% AHAs).
Ascorbic acid (to prevent complications)
Side effects of HQ include allergic and contact dermatitis (more likely at higher concentrations), post-inflammatory hyperpigmentation, and nail discoloration. These effects are transient and resolve with discontinuation of HQ. A very rare complication - ochronosis (permanent blue-black post-inflammatory pigmentation of the skin) - is seen in individuals of African descent.
Combination of hydroquinone with other substances
It has been shown that the skin lightening effect attributed to HQ can be enhanced by the addition of various topical agents such as tretinoin and corticosteroids. The following combination (Kligman and Willis formula) has been suggested:
- 5% HQ;
- 0.1% tretinoin;
- 0.1% dexamethasone;
- In ethanol and propylene glycol 1:1 or in a hydrophilic ointment.
Tretinoin stimulates cellular turnover, facilitating rapid pigment loss through epidermoforesis. It also acts as a mild irritant, aiding the epidermal penetration of HQ. Tretinoin acts as an antioxidant, preventing HQ oxidation. Corticosteroids can slow down melanin synthesis by suppressing overall cell metabolic activity. In addition, they may reduce irritation caused by HQ and/or tretinoin. Depigmentation begins within 3 weeks of twice-daily application for up to 5-7 weeks. This formula cannot be preserved with antioxidants and should not be used if it's older than 30 days.
A slight modification of the Kligman and Willis formula:
- 4% HQ;
- 0.05% tretinoin;
- 1% hydrocortisone acetate;
- In ethanol and propylene glycol 1:1 or in a hydrophilic ointment.
This formula uses 0.05% tretinoin and 1% hydrocortisone instead of dexamethasone. The reduction in tretinoin concentration and the use of a non-fluorinated steroid are intended to minimize the irritation caused by tretinoin and to eliminate local steroid effects. These two formulas should be stored in a 25 ml dark glass bottle with a tight cap in the refrigerator at a temperature of 2-3°C.
Another therapeutic approach is the sequential application of 0.05% tretinoin cream, 2% HQ and 1% hydrocortisone acetate during the day.
Azelaic Acid (AA)
AA is a naturally occurring straight-chain saturated dicarboxylic acid. It has been reported that AA acts as a competitive inhibitor of tyrosinase and directly interferes with melanin biosynthesis. AA has no effect on normal melanocytes. AA cream is effective in the treatment of melasma. It is applied twice daily and most patients report mild transient irritation and dryness at the beginning of treatment. Various studies report good to excellent results in 63-80% of patients with epidermal or mixed melasma after 6 months of treatment with 20% AA cream in combination with a broad-spectrum sunscreen. As mentioned above, AA has minimal effect on normal melanocytes and its long-term use is not associated with ochronosis. Since treatment of patients with AA requires several months, another therapeutic option is to combine AA with other agents.
Chemical Peels
Superficial and moderate chemical peels are used to treat melasma in fair-skinned individuals. All types of chemical peels, especially trichloroacetic acid (TCA) and AHAs at various concentrations, have been used both as monotherapy and in combination with other depigmenting agents. However, it's important to note that the response of melasma to chemical peels is quite unpredictable: there is a tendency for pigment changes, but in darker-skinned individuals, melasma may worsen after chemical peeling due to post-inflammatory hyperpigmentation.
Alternative and Experimental Treatment Methods
Laser Treatment
In recent years, various types of lasers have been tried for the treatment of melasma, but unfortunately with limited success. While specific lasers designed for pigmented lesions with pulse duration modulation produced excellent results in the treatment of lentigo-spots, their use in melasma proved ineffective and often resulted in hyperpigmentation. The initial use of a ruby laser with pulse duration modulation showed some positive results, especially in patients with fair skin and epidermal-type melasma. However, there was a rapid relapse after discontinuation of treatment. Since laser treatment is not without side effects (hyperpigmentation, minor scarring, atrophy, etc.), the role of lasers in the treatment of melasma is still under investigation. Encouraging results have been observed in the treatment of the more persistent dermal-type melasma by laser surface correction (fractionated CO2 or erbium lasers) to remove superficial skin, including abnormal melanocytes. Patients with stubborn melasma treated with fractionated erbium (2940 nm) lasers experienced significant lightening of pigmentation at 6 months, despite initial post-inflammatory hyperpigmentation in almost all patients. Another approach to the treatment of dermal melasma involves CO2 laser surface treatment followed by alexandrite laser application with pulse duration modulation to selectively target dermal melanin.
Kojic Acid
Kojic acid is a fungal metabolic product that inhibits tyrosinase catalytic activity. It has been used as monotherapy at concentrations of 2-4% and in combination with a 2% HQ gel containing AHAs.
Tranexamic Acid
This compound also has the ability to block pigment production through tyrosinase inhibition. It is available for both topical and oral administration and may be considered for patients who have not responded to hydroquinone or other combined topical agents. Tranexamic acid may also be used as maintenance therapy after hydroquinone has achieved its effects. It is contraindicated in patients with deep vein thrombosis or those taking oral contraceptives because it increases blood clotting. The standard dose is 250-500 mg twice daily. Topical treatment is performed with solutions of 2% or 3% concentration.
Cysteamine
A non-hydroquinone topical cosmetic derived from cysteine. A 5% cream contains cysteamine hydrochloride, which inhibits melanin synthesis. It is applied as a mask and then rinsed off.
Heliocare
An oral antioxidant containing fern leaf extract, Polypodium leucotomos, gelatin, lutein, lycopene, vitamins E, D, C; E171, E172, E132, which provides additional protection against free radicals in sunlight, infrared and visible light that can damage the skin. It is taken as 1 capsule a day for 1-2 months. The efficacy of all the substances listed above requires further research.